Virtual Research Office
For Medical Center Investigators

Jiaxiao Shi, Biostatistics Manager; Nirupa Ghai, Research Navigator; and Stephanie Tovar, Program Manager
The Southern California Permanente Medical Group (SCPMG) supports clinicians interested in conducting research with several programs.
Priority is given to projects that are aligned with Kaiser Permanente’s mission and strategic priorities as articulated and communicated by our senior SCPMG leaders and can have important impacts on our clinical operations or improve our members’ lives.
If you’re a physician, nurse, pharmacist, anesthesiologist, health educator, or other Kaiser Permanente Southern California clinician who’s interested in conducting research, the information and resources below will help you get started.
QUICK LINKS
Interested researchers, please see these quick links and general information below about research opportunities, how to do a research projects, applying for funding, and frequently asked questions.
- Submit a request to set up a consultation. From outside the Kaiser Permanente network, you’ll need to click the access link and may need to use your PingID.

- View the training document for medical center investigators on how to submit a VRO request and training video.
- Contact our research navigator, Nirupa Ghai, PhD, MPH.
She can help you to understand the research process, explore funding options, and get your projects started.
Research opportunities for Kaiser Permanente clinicians
- Some of the best research opportunities are available via collaboration with one of the full-time research scientists in the Department of Research & Evaluation (R&E). You’ll find scientists’ areas of expertise in their online profiles.
- Clinician-led projects are also encouraged. Education Time (ET one-half day) is part of the SCPMG benefit that allows physicians to conduct research.
- Internal or external funding sources generally support programming/analytic time, research project manager, research associate time, and often physician time, especially for large-scale projects.
- Information about resident and fellow research is available on the Graduate Medical Education website.
- Clinical trials: KPSC clinicians can learn more about clinical trials here.
Outside activity opportunities for SCPMG physicians
- Outside Activity Approval Process for SCPMG Physicians Who Participate in Research information is available by visiting inside the Kaiser Permanente network and clicking here.
Competitive sources of internal (KPSC) funding include:
- The Regional Research Committee (RRC, led by Bechien Wu, MD) provides grants from $8,000 to $55,000 for 1-year physician-initiated projects. Applications are received and reviewed 2 times per year. The RRC solicits letters of intent and provides consultation services to applicants regarding their research projects. Proposals are reviewed by content experts as well as the committee. Proposals are assigned priority scores based on clinical significance, innovation, and feasibility. Critical feedback is provided to all applicants regardless of funding status. Physicians are encouraged to discuss potential projects with their local area research chair prior to submission.
- Care Improvement Research Team (CIRT, co-led by Huong Nguyen, PhD, RN, and Bechien Wu, MD, MPH) provides grants from $20,000 to $100,000 for 1-year projects with aims to improve access, quality, and affordability of care delivery and the health of patients, families, and communities. The annual funding cycle includes letters of intent and proposals submitted during the summer and fall, with funding granted in November for the following year. Additional projects may be funded at other times during the year for work supported by senior leadership.
- Clinician Investigator Program (CIP, led by William Towner, MD) provides a more formal career path for physicians with prior research experience wanting to make research an integral part of their careers. Two-year (renewable) appointments are made each year. Support includes administrative support (0.25 FTE), statistical support (0.25 FTE) and 2/10 FTE (1 FTE of which will be the physicians Educational Time (ET) and is subject to medical center approval) protected time for the physician.
- The Garfield Memorial Fund (GMF) provides financial support for research and demonstration projects within Kaiser Permanente, with an emphasis on efforts in support of Kaiser Permanente’s National Clinical Quality Strategy – provision of new services to members, optimization of existing services, analyses of members’ needs, and innovation in meeting those needs. The GMF accepts applications in response to themed calls for applications as well as investigator-initiated proposals.
External funding is available via federal (e.g., NIH, AHRQ, PCORI), private (e.g., foundations, professional societies), and local (e.g., city, state, industry) agencies. R&E is available to facilitate the preparation, development, and submission of proposals. R&E also negotiates and executes contracts, provides post award stewardship, provides the necessary staffing to carry out the aims of a research project.
Support for unfunded projects available to all physicians includes:
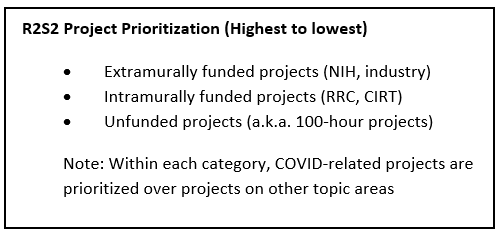
- R2S2 (Regional Research Statistical Services) Team within R&E (led by Jiaxiao Shi, PhD) provides up to 100 hours of programming and analytic time to interested medical center researchers for projects of limited scope that can be accomplished primarily using existing data. Investigators may have only one active project at a time supported by R2S2. To access these services, submit a request here inside the Kaiser Permanente network.
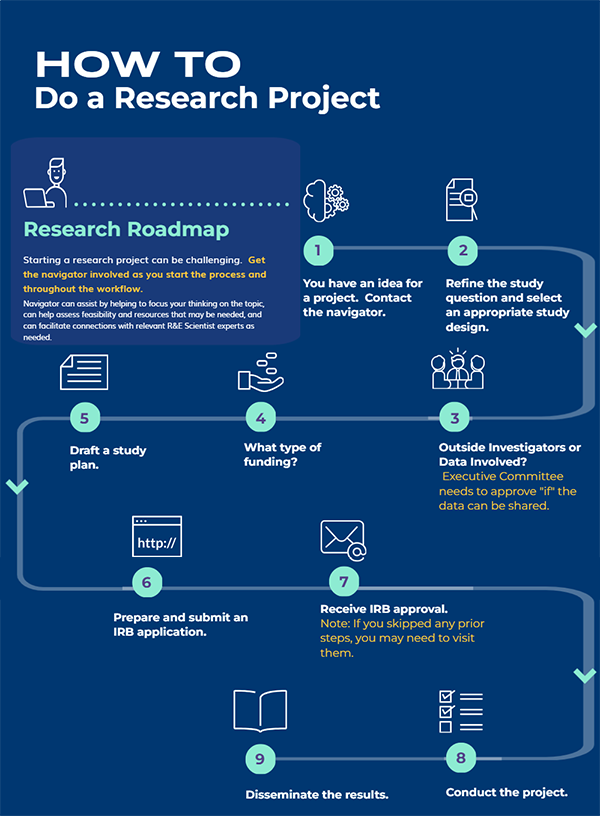
STEP 1: You have an idea for a project.
Conduct a thorough literature review to see if your topic has been addressed. The clinical librarians can assist with the search.
Write down your initial version of a research question and a brief (1-page) summary of your ideas, including a statement of why answering this research question is important (overall and to Kaiser Permanente specifically).
Get Nirupa Ghai, PhD, MPH, the navigator, involved as early as possible. She can assist by helping to focus your thinking on the topic, can help assess feasibility and resources that may be needed, and can facilitate connections with relevant R&E scientist experts as needed.
STEP 2: Refine your study question into one that is FEASIBLE, INTERESTING, NOVEL, ETHICAL, AND RELEVANT, and select an appropriate study design.
Think about ways that the answer to the question might impact our delivery of care to our members and/or change our medical practice.
Keep it focused and don’t be too ambitious – a rigorous answer to a focused question is better than less rigorous attempts to answer multiple questions simultaneously. You can always address additional questions in subsequent projects.
This process may involve other parties such as research scientists, R2S2, etc. The navigator can coordinate these connections.
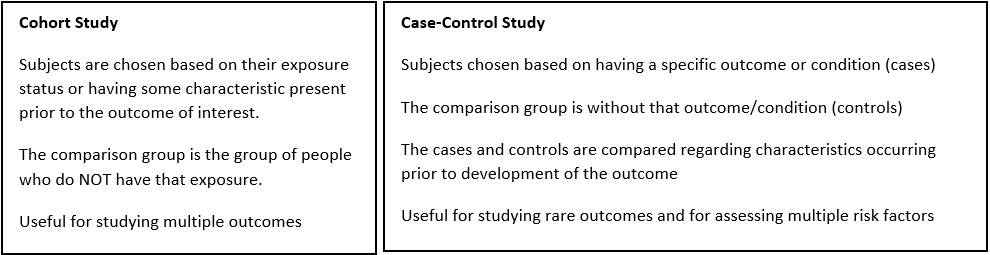
Choose a study design.
Contact the navigator, who is an expert in refining research questions and choosing appropriate study designs. Send the summary page from Step 1 to the navigator for review prior to consultation.
STEP 3: Will your proposed project involve any investigators outside of Kaiser Permanente?
If yes, understand that, in general, no patient-level data will be obtainable/viewable by outside investigators. Only aggregated results, like results tables for manuscripts, will be allowed to be shared externally.
Will your proposal include transfer of KPSC data to an outside organization/institution?
If yes, please review this memo. Please contact Nirupa Ghai, PhD, MPH, the navigator, to discuss. Please note the R&E Executive Committee must pre-approve any data disclosed outside of KPSC. IRB approval of a project is secondary to R&E Executive Committee approval. Make sure all prior approvals are completed before submitting an IRB application. To get R&E approval please fill out a request in the Disclosure and Funding Authorization application.
View the KPSC Collaborative Research Policy.
STEP 4: Decide if you will pursue internal or external funding or if the project will be an unfunded project.
This decision will necessarily affect the feasible scope of the project.
The navigator can provide advice about whether you need funding to accomplish your project, and if so, can provide thoughts about the appropriate funding sources to consider.
For unfunded projects, MCIs have access to 100 hours of programming and biostatistics effort from the R2S2 group (supported by R&E). The scope must be narrow enough to be accomplished within this level of effort. This is ideal for data-only studies addressing focused research questions. To access these services, submit a request here inside the Kaiser Permanente network.
For funded projects, there are additional steps required as outlined in the R&E General Steps for Applying for External Funding. In addition, administrative assistance is available to support applications for external funding.
STEP 5: Draft a preliminary full-length study plan/protocol.
Once the best study design has been chosen, you can begin writing the full-length study plan/protocol.
Templates are available to provide guidance and to prompt your thinking about details necessary for conduct of the study.
As you write the study plan, try to be as detailed as possible. Clearly define how subjects will be identified and chosen (ICD codes, specific procedures, pharmacologic use?). Include relevant details like the codes or medication names, etc.
Please use the Nirupa Ghai, PhD, MPH, the navigator, as a resource here – the navigator can review drafts, provide suggestions, and ask probing questions to prompt your thinking.
STEP 6: Prepare and submit IRB application.
This can be done in parallel with developing the Study Plan. Make sure all prior approvals are completed before submitting an IRB application. If you plan to disclose data outside of KPSC, please fill out a request for approval in the Disclosure and Funding Authorization application.
If you have never led a research project before, you will need to:
Get an iRIS account – allows access to the IRB’s online application submission portal: irissupport.kp-scalresearch.org inside the Kaiser Permanente network.
Complete the required research trainings: irb.kp-scalresearch.org/irbtraining.html
The main IRB website is located here: irb.kp-scalresearch.org
The IRB also has 2 excellent points of contact for questions that arise regarding obtaining approval for your project: Marcela Sanchez and Daria Diaz.
Pro-tip: The IRB application requires less detailed information than is needed in the Study Plan, but an IRB application does not suffice as the sole study plan document (not enough detail to actually *do* the study). Much of what you’re writing in the study plan can be copied/pasted into the IRB application to minimize double work. And much of what is written in the full-length study plan can be recycled and used as text in your subsequent manuscript.
The navigator can provide some guidance about completing the IRB application and can review drafts, etc.
Step 7: Once the project is IRB approved, data can only be released after executive committee approval and full execution of a data use agreement.
Please fill out a request for approval in the Disclosure and Funding Authorization application.
Once the project is IRB approved, contact Nirupa Ghai, PhD, MPH, the navigator, to make sure you’re in the queue for programmer/biostat availability to work on your project. There is often a wait of a few weeks between IRB approval and initiation of your project by R2S2.
Step 8. Conduct the project.
Projects often take a few months once work has begun. Programmers/biostatisticians are not able to devote full effort toward any single project at any time.
Work with the programmer/biostatistician will be interactive and iterative. They may have clarifying questions for you or need you to review some things for validation of case ascertainment, etc. The biostatistician will provide results at various stages and assist with interpretation.
Step 9. Disseminate the results.
Your project isn’t complete until the results have been disseminated to the broader scientific community via abstract presentation at a meeting and/or publication of a manuscript.
Coaching on abstract and manuscript writing is available – whomever will be first author is expected to do the majority of the actual writing.
Resources are available to assist with formatting/printing posters and formatting manuscripts.
Caveat
If you have a deadline for completion of the project, you will need to begin this process months in advance. Consider the following:
- time it takes for you to develop a proposal of sufficient quality for IRB submission and for conduct of the project
- time for IRB review (several weeks)
- wait time for available programmer/analyst
- time for you to conduct any necessary manual chart reviews
- time for programmer to extract and clean data
- time for biostatistician to conduct analyses
- time for you to summarize the findings


